lv values kj kg | 13.3: Phase Change and Latent Heat lv values kj kg Values are usually quoted in J/mol, or kJ/mol (molar enthalpy of vaporization), although kJ/kg, or J/g (specific heat of vaporization), and older units like kcal/mol, cal/g and Btu/lb are sometimes still used among others. Gamechanger Audio. Tomsona str 33A. Riga, LV-1013. Latvia. News and subscriber-only deals! Name . Email . Subscribe. Curious? Leave your email to receive news and subscriber-only deals! Name .
0 · Phase Change and Latent Heat
1 · Liquids
2 · Latent heat
3 · Latent Heat of Water
4 · Latent Heat Calculator
5 · Give the values for the latent heat of vaporization and latent heat
6 · General Physics II
7 · Enthalpy of vaporization
8 · Energy Matters – Heat Changes of State
9 · 13.3: Phase Change and Latent Heat
LOUIS VUITTON Official site United Kingdom - Explore the World of Louis Vuitton, Purchase online our Women and Men Collections and locate our Stores.
Phase Change and Latent Heat
The latent heat of the fusion of 5 kg of water is 1670 kJ. To find this number on your own, you need to multiply the specific latent heat of the fusion of water ( 334 kJ/kg ) times the mass of the water ( 5 kg ).
Liquids
Values are usually quoted in J/mol, or kJ/mol (molar enthalpy of vaporization), although kJ/kg, or J/g (specific heat of vaporization), and older units like kcal/mol, cal/g and Btu/lb are sometimes still used among others.The units of Latent Heat of Vaporisation are J/kg. L v is the heat energy required to change 1kg of a liquid (at boiling point) into 1kg of gas (at condensing point).
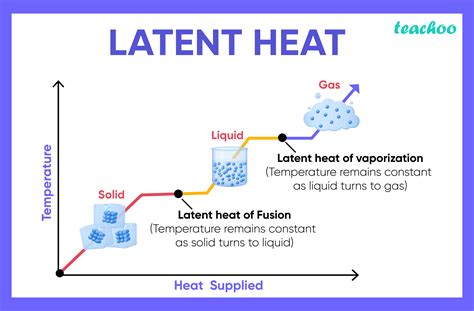
A specific latent heat (L) expresses the amount of energy in the form of heat (Q) required to completely effect a phase change of a unit of mass (m), usually 1kg, of a substance as an intensive property: Intensive properties are material characteristics and are not dependent on the size or extent of the sample. Commonly quoted and tabulated in the literature are the specific latent heat of fusion a.Latent heat of fusion of water = 79.5 cal/g = 333 kJ/kg. Specific heat of water = 1 cal/g = 4.19 kJ/kg. Similarly, while ice melts, it remains at 0 °C (32 °F), and the liquid water that is formed with the latent heat of fusion is also at 0 °C.Latent heat is an intensive property measured in units of J/kg. Both L f and L v depend on the substance, particularly on the strength of its molecular forces as noted earlier. L f and L v are collectively called latent heat coefficients.
Latent heat is measured in units of J/kg. Both Lf and Lv depend on the substance, particularly on the strength of its molecular forces as noted earlier. Lf and Lv are collectively called latent heat coefficients.
The latent heat of evaporation for water is 2256 kJ/kg at atmospheric pressure and 100 o C. The heat required to evaporate 10 kg can be calculated as q = (2256 kJ/kg) (10 kg)The heat of fusion for ice or water is L f = 3.33 x 10 5 J/kg. The specific heat of (frozen) ice is ci = 2090 J/ (kg Co). Notice that the specific heat is different for ice and water! Working many examples is the best way to become comfortable with Equilibrium Temperature problems.
Latent heat
Latent Heat of Water
rolex bestellen uit china
L is specific latent heat for a particular matter (kJ kg-1); Lv for vaporization and Lf for fusion. Note: The latent heat of water at 0 degree Celsius for fusion is nearest to 334 joules per gram or 79.7 calories per gram.
The latent heat of the fusion of 5 kg of water is 1670 kJ. To find this number on your own, you need to multiply the specific latent heat of the fusion of water ( 334 kJ/kg ) times the mass of the water ( 5 kg ).
Values are usually quoted in J/mol, or kJ/mol (molar enthalpy of vaporization), although kJ/kg, or J/g (specific heat of vaporization), and older units like kcal/mol, cal/g and Btu/lb are sometimes still used among others.The units of Latent Heat of Vaporisation are J/kg. L v is the heat energy required to change 1kg of a liquid (at boiling point) into 1kg of gas (at condensing point).
Q is the amount of energy released or absorbed during the change of phase of the substance (in kJ or in BTU), m is the mass of the substance (in kg or in lb), and. L is the specific latent heat for a particular substance (in kJ kg −1 or in BTU lb −1), either Lf for fusion, or Lv for vaporization.Latent heat of fusion of water = 79.5 cal/g = 333 kJ/kg. Specific heat of water = 1 cal/g = 4.19 kJ/kg. Similarly, while ice melts, it remains at 0 °C (32 °F), and the liquid water that is formed with the latent heat of fusion is also at 0 °C.
Latent heat is an intensive property measured in units of J/kg. Both L f and L v depend on the substance, particularly on the strength of its molecular forces as noted earlier. L f and L v are collectively called latent heat coefficients.Latent heat is measured in units of J/kg. Both Lf and Lv depend on the substance, particularly on the strength of its molecular forces as noted earlier. Lf and Lv are collectively called latent heat coefficients. The latent heat of evaporation for water is 2256 kJ/kg at atmospheric pressure and 100 o C. The heat required to evaporate 10 kg can be calculated as q = (2256 kJ/kg) (10 kg)
The heat of fusion for ice or water is L f = 3.33 x 10 5 J/kg. The specific heat of (frozen) ice is ci = 2090 J/ (kg Co). Notice that the specific heat is different for ice and water! Working many examples is the best way to become comfortable with Equilibrium Temperature problems.L is specific latent heat for a particular matter (kJ kg-1); Lv for vaporization and Lf for fusion. Note: The latent heat of water at 0 degree Celsius for fusion is nearest to 334 joules per gram or 79.7 calories per gram. The latent heat of the fusion of 5 kg of water is 1670 kJ. To find this number on your own, you need to multiply the specific latent heat of the fusion of water ( 334 kJ/kg ) times the mass of the water ( 5 kg ).
Values are usually quoted in J/mol, or kJ/mol (molar enthalpy of vaporization), although kJ/kg, or J/g (specific heat of vaporization), and older units like kcal/mol, cal/g and Btu/lb are sometimes still used among others.The units of Latent Heat of Vaporisation are J/kg. L v is the heat energy required to change 1kg of a liquid (at boiling point) into 1kg of gas (at condensing point).Q is the amount of energy released or absorbed during the change of phase of the substance (in kJ or in BTU), m is the mass of the substance (in kg or in lb), and. L is the specific latent heat for a particular substance (in kJ kg −1 or in BTU lb −1), either Lf for fusion, or Lv for vaporization.
Latent heat of fusion of water = 79.5 cal/g = 333 kJ/kg. Specific heat of water = 1 cal/g = 4.19 kJ/kg. Similarly, while ice melts, it remains at 0 °C (32 °F), and the liquid water that is formed with the latent heat of fusion is also at 0 °C.
Latent heat is an intensive property measured in units of J/kg. Both L f and L v depend on the substance, particularly on the strength of its molecular forces as noted earlier. L f and L v are collectively called latent heat coefficients.
Latent heat is measured in units of J/kg. Both Lf and Lv depend on the substance, particularly on the strength of its molecular forces as noted earlier. Lf and Lv are collectively called latent heat coefficients. The latent heat of evaporation for water is 2256 kJ/kg at atmospheric pressure and 100 o C. The heat required to evaporate 10 kg can be calculated as q = (2256 kJ/kg) (10 kg)
The heat of fusion for ice or water is L f = 3.33 x 10 5 J/kg. The specific heat of (frozen) ice is ci = 2090 J/ (kg Co). Notice that the specific heat is different for ice and water! Working many examples is the best way to become comfortable with Equilibrium Temperature problems.
Latent Heat Calculator
Give the values for the latent heat of vaporization and latent heat
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright .
lv values kj kg|13.3: Phase Change and Latent Heat